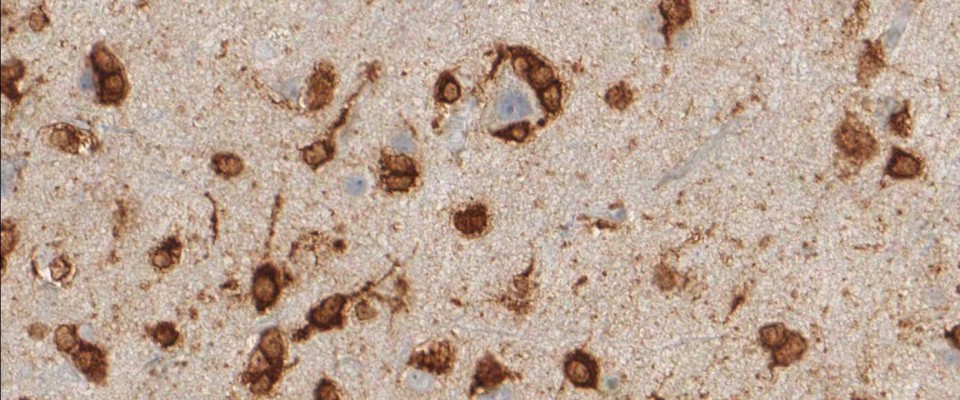
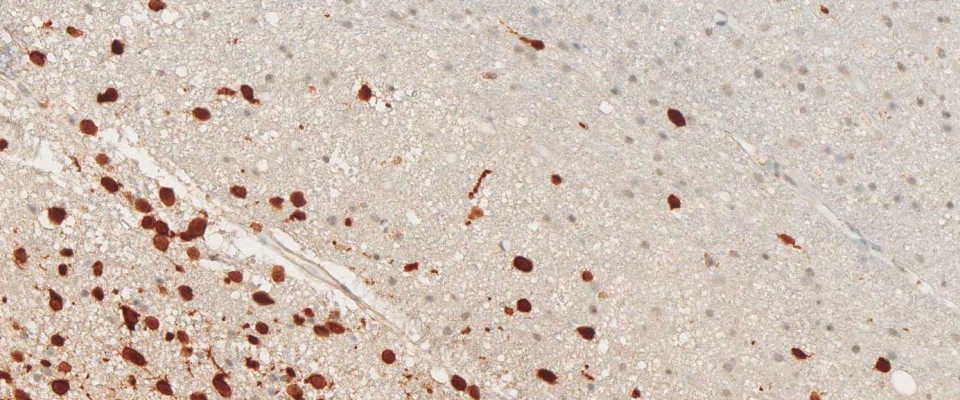
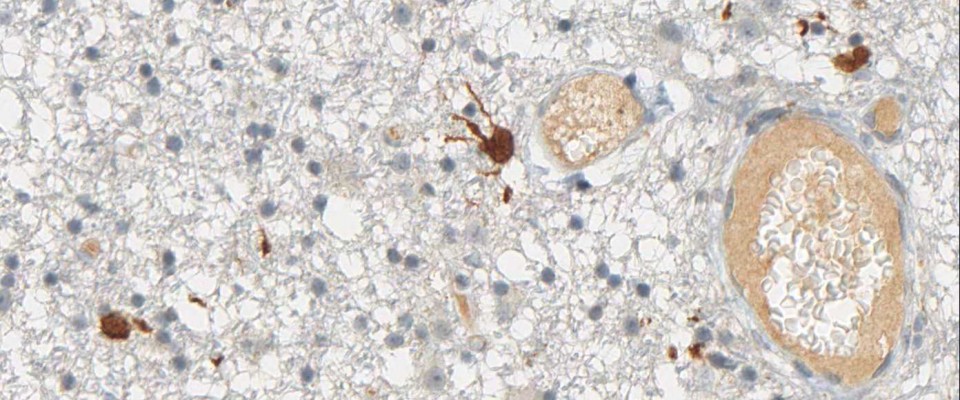
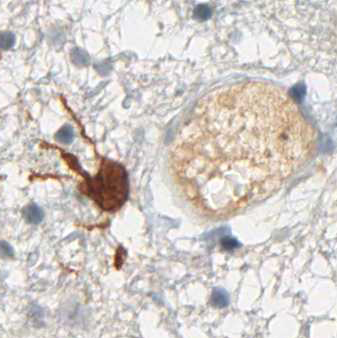
The immunohistochemical analysis of the IDH1 R132H mutation can be performed manually or by automated immunostaining procedures. Protocol recommendations have been published in several studies and practical guidelines and allow satisfactory results in the routine immunohistochemistry of formalin-fixed, paraffin-embedded (FFPE) tissues (1,2,3). The National Comprehensive Cancer Network (NCCN) and EURO-CNS research committee recommend to first perform immunohistochemical testing with the anti-IDH1 R132H antibody, and only in case of negative staing results to follow up with DNA/PCR-based detection techniques (1,2).
Ventana Autostainer
Procedure: Automated Immunostaining Ventana Benchmark®XT XT ultraView DAB
Summary
- Cut sections to 4 μm (Microm HM 355 S) and dry at 80° C for 15 min.
- Dilute anti-IDH1 R132H antibody clone H09 1:20-1:50 (antibody diluent from Ventana) and fill into a Ventana antibody dispenser.
- The Ventana staining procedure includes pretreatment with Cell Conditioner 2 (pH 6) for 60 min (standard), followed by incubation with 1:20-1:50 diluted antibody clone H09 at 37 °C for 32 min.
- Upon antibody incubation perform Ventana standard signal amplification, ultraWash, counter- staining with one drop of Hematoxylin for 4 min and one drop of bluing reagent for 4 min.
- For chromogenic detection use ultraView Universal DAB Detection Kit (Ventana)
- Remove slides from stainer, wash in water with a drop of dishwashing detergent and mount.
Important note:
Diffuse astrocytoma WHO grade II may have low protein-expression.
At high dilution of the antibody single tumor cells in the infiltration zone may not be stained.
Ventana Short Protocol
- paraffin [selected]
- dewaxing [selected]
- heat pretreatment [selected]
- Cell Conditioner 2 [selected]
- Mild CC2 [selected]
- Standard CC2 [selected]
- define antibody incubation temperature [selected]
- 37°C [selected]
- antibody [selected]
- apply 1 drop [PREP KIT 101] (antibody), incubate for [0 h 32 min]
- amplify [selected]
- ultraWash [selected]
- counterstaining [selected]
- apply 1 drop [HEMATOXYLIN] (counterstaining), apply LCS and incubate for [4 min]
- after-counterstaining [selected]
- apply 1 drop [BLUING REAGENT] (after-counterstaining), apply LCS, incubate for [4 min]
Ventana Full Protocol
- ***** select EZPrep *****
- ***** start timed steps *****
- ***** mixer off *****
- heat object slide up to 75°C and incubate for 4 min
- balance EZPrep volume
- wash slide
- balance EZPrep volume
- wash slide
- balance EZPrep volume
- apply coverslip
- heat slide up to 75°C and incubate for 4 min
- wash slide
- balance dewaxing volume
- apply coverslip
- turn off slide heater
- ***** mixer on *****
- [short 8-minute-conditioning]
- wash slide
- apply Cell Conditioner No. 2 long
- release of Cell Cond. and Coverslip, long
- ***** select SSC Wash *****
- heat slide up to 94°C and incubate for 8 min
- [mild 36-minute-conditioning]
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- heat slide up to 95°C and incubate for 4 min
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- add EZPrep CC Volume Adjust
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- [standard 60-minute-conditioning]
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- add EZPrep CC Volume Adjust
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- apply Cell Conditioner No. 2
- apply Cell Cond. and Coverslip (without barcode blowoff)
- turn off slide heating
- incubate for 8 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- ***** synchronise procedures *****
- heat slide up to 37°C and incubate for 4 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop UV INHIBITOR and coverslip, and incubate for 4 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- heat slide up to 37°C and incubate for 4 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- apply 1 drop [PREP KIT 101] (antibody) and incubate for [0 h 32 min]
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- heat slide up to 37°C and incubate for 4 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop AMPLIFIER A and coverslip, incubate for 8 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop AMPLIFIER B and coverslip, incubate for 8 min
- rinse with reaction buffer
- add 200 ml and balance reaction buffer volume
- apply 1 drop UV HRP UNIV MULT and coverslip, incubate for 8 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop UV DAB and 1 drop UV DAB H2O2 and LCS and incubate for 8 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop UV COPPER and coverslip, incubate for 8 min
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop [HEMATOXYLIN] (counterstaining) and LCS, and incubate for [4 min]
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply coverslip
- rinse with reaction buffer
- fine adjustment of reaction buffer volume
- apply 1 drop [BLUING REAGENT] (after-counterstaining) and LCS, and incubate for [4 min]
- rinse with reaction buffer
- apply coverslip
- turn off slide heater
- ***** select optional washing procedure *****
- ***** select SSC Wash *****
- ***** start timed steps *****
- rinse with reaction buffer
DAKO Autostainer
Procedure: Automated Immunostaining DAKO EnVisionTM FLEX
Summary
- Dewax and rehydrate 4 µm paraffin-embedded tissue sections.
- Perform antigen retrival using EnVisionTM FLEX target retrival solution at pH10 for 20min at 95°C.
- Cool slides and treat with EnVisionTM FLEX peroxidase-blocking reagent solution for 5min.
- Incubate sections with anti-IDH1 R132H/clone H09 primary antibody at 1:20 dilution in EnVisionTM FLEX antibody diluent for 20min.
- Complete immunostainig by EnVisionTM FLEX + Mouse (LINKER) / HRP technique following manufacturer’s instructions.
- Counterstain with hematoxylin and mount.
Important note:
Diffuse astrocytoma WHO grade II may have low protein-expression.
At high dilution of the antibody single tumor cells in the infiltration zone may not be stained.
DAKO EnVisionTM FLEX Protocol
- Dewax & rehydrate sections
- Heat pretreatment: EnVisionTM FLEX target retrival solution, 95°C, 20min.
- Cool slides
- Rinse with buffer 2x
- Endogenous enzyme block: EnVisionTM FLEX peroxidase-blocking reagent, 5min.
- Rinse with buffer 1x
- Primary antibody: anti-IDH1 R132H/clone H09, 1:20 in EnVisionTM FLEX antibody diluent, 20min.
- Rinse with buffer 1x
- Secondary Reagent: EnVisionTM FLEX + Mouse (LINKER), 15min.
- Rinse with buffer 1x
- Labelled Polymer: EnVisionTM FLEX / HRP, 20min.
- Rinse with buffer 2x
- Substrat-Chromogen: Substrate working Solution (mix), 10min.
- Rinse with buffer 1x
- Counterstain: EnVisionTM FLEX Hematoxylin, 5min.
- Rinse with buffer 1x
- Mount
Manual Staining
Procedure: Immunostaining performed manually HRP/DAB
Polymer Detection kit
- Dewax and rehydrate sections: Xylol: 3x10min / EtOH: 2×100%, 2x 95%, 1×70%, 1xH2O; 3min each.
- Perform heat induced antigen retrival (HIER) using citrate buffer at pH6 (CC2 solution, Ventana) by cooking for 60min in a steamer.
- Cool slides for 5min.
- Wash with 3 changes of PBS buffer, 3min incubation per step
- Blocking endogenous peroxidases: Place slides in Peroxidase-blocking solution for 10min at RT.
- Wash with 3 changes of PBS buffer, 3min incubation per step
- Blocking: Place slides in PBS buffer with 5% FCS and incubate for 30min at RT.
- Cover tissue with primary antibody anti-IDH1 R132H/clone H09:
Dilute 1:20-1:40 in PBS with 5% FCS and incubate at 4°C over night. - Wash with 3 changes of PBS buffer, 3min incubation per step
- Secondary antibody: Cover tissue with Anti-mouse/rabbit polymer HRP-label for 30min at RT
- Wash with 3 changes of PBS buffer, 3min incubation per step
- Prepare DAB by adding 2 drops of DAB-chromogen per 1ml DAB-substrate buffer and mix
- Staining reaction: Cover tissue with prepared DAB chromogen solution, incubate approximately for 10min. to allow for proper brown colour development.
- Wash slides thoroughly in H2O
- Counterstain with hemalaun for 2min
- Wash slides in H2O
- Coverslip with mounting medium (Immunoselect, dianova)